EXPERIMENTAL STUDY ON THE INHIBITORY EFFECT OF EXPIRED CHLORPROMAZINE ON CORROSION OF MILD STEEL IN ACIDIC MEDIUM
Keywords:
Corrosion inhibition, Chlorpromazine, Mild steel, H2SO4, Adsorption, ThermodynamicsAbstract
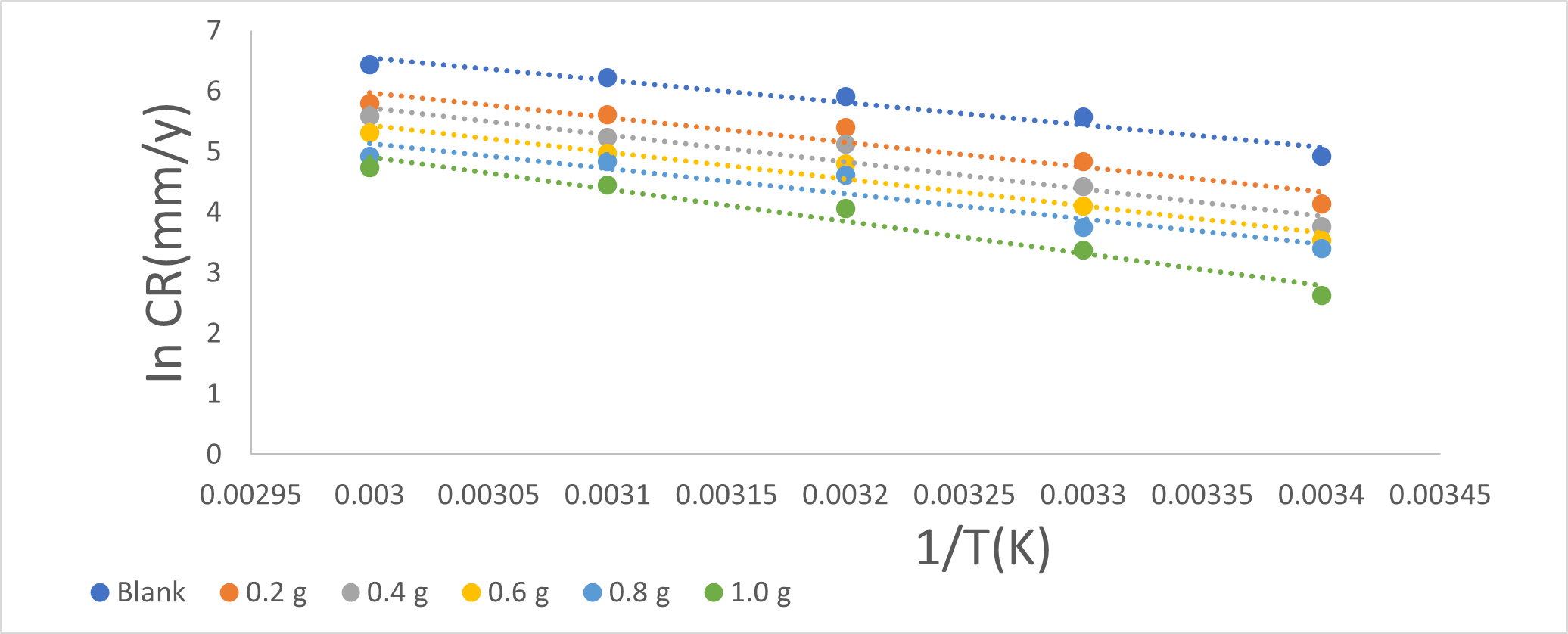
Chlorpromazine was investigated for its corrosion inhibition potential on mild steel in 0.5 M H2SO4. Weight loss measurement and electrochemical impedance spectroscopy (EIS) methods were used. The concentrations of chlorpromazine ranges from (0.2 g to 1.0 g) and temperatures (298 – 333 K) at 1 hour on corrosion in acid medium and corrosion inhibition was assessed. The results showed that chlorpromazine decreased the corrosion rate of mild steel in the acid media. The rate generally decreased with increasing the concentration of chlorpromazine in acidic medium for mild steel at different temperature (298 K-333 K) at 1 hour. The maximum inhibition efficiency was 93.66 % at 298 K 0.5 M H2SO4 for 1 h on mild steel. As the temperature increases the inhibition efficiency decreases (333 K), suggesting physical adsorption of the chlorpromazine constituents on the metal surfaces. Electrochemical impedance spectroscopy was used as a method to investigated chlorpromazine in acidic medium. As the concentration of the expired chlorpromazine increases, the radius and size of semicircles increases. This demonstrates that the characteristics of the coating film on the surface of the mild steel increased thereby reduced the corrosion rate.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Femi Emmanuel Awe; Mohammed Ya'u, Saibu Muyiwa

This work is licensed under a Creative Commons Attribution 4.0 International License.




